Mammalian GMP Manufacturing for Biologics
With its multi-scale mammalian GMP facilities, GTP Bioways is the ideal CDMO partner to support your clinical development with the manufacturing of biotherapeutics, vaccines and ancillary reagents.
Our mammalian GMP production capacities for clinical & commercial supply
Saint-Julien facility
The innovative design of our state-of-the art facilities allows for capacity and technological flexibility, while ensuring compliance with the strict biopharmaceutical regulations required for cGMP operations.
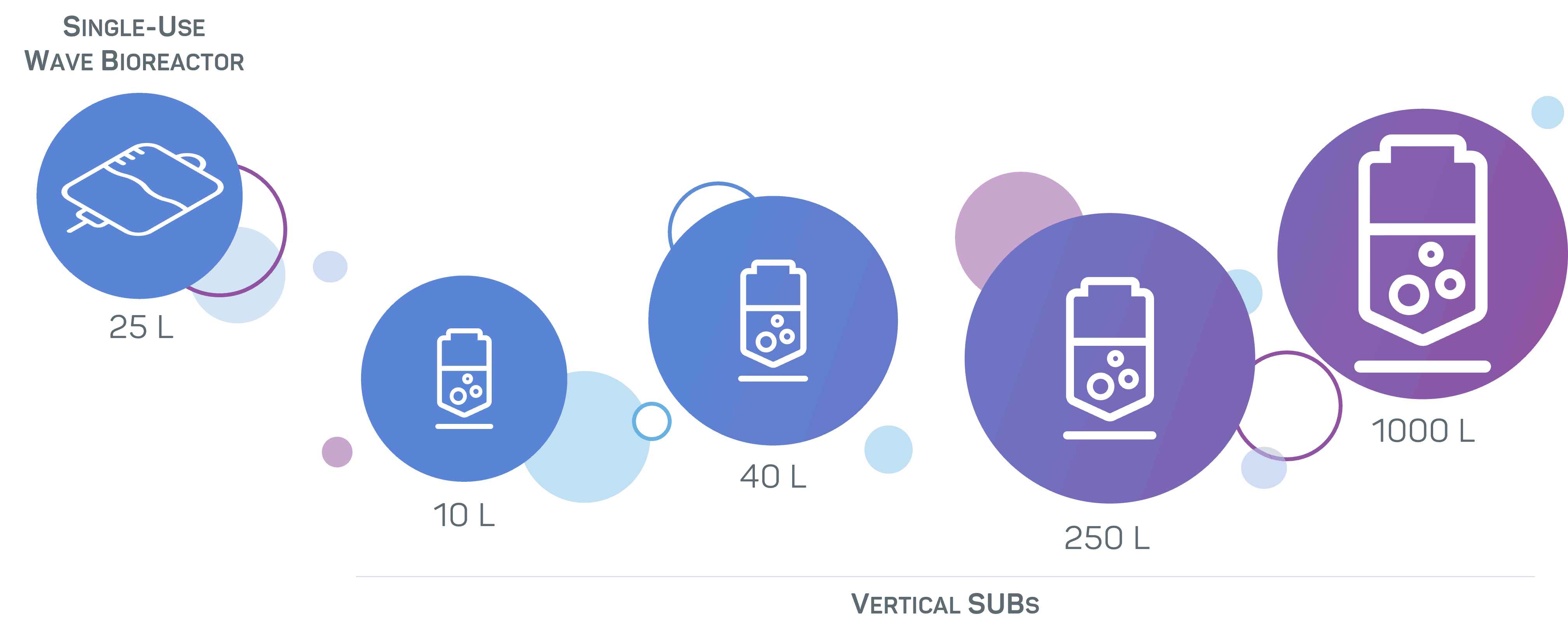
With production unit capacities ranging from 250 L to 1000 L, we can manufacture up to kilogram batches. Our suites are equipped with single-use technologies for the flexible production of your biologic with rapid turnaround times.

Toulouse facility
In order to offer an optimised cost per gram for applications that require small quantities of GMP material, we have designed a mammalian manufacturing facility for the production of quantities from a few milligrams up to tens of grams of recombinant proteins or monoclonal antibodies. This suite is ideally sized in particular for the manufacturing of ancillary reagents for cell therapy.
This top-notch cGMP manufacturing line is fully equipped with single-use technologies, to offer great flexibility:
- Fully single-use vertical and wave bioreactors with working volumes ranging from 10 to 40 L.
- Flexible chromatography equipment able to process reduced quantities (5 to 1200 ml/min)
- TFF systems ranging from 0.1 m² to 2 m² meeting the requirements for small batch production.


