A technology transfer (or CDMO tech transfer) consists in transferring process knowledge from development to manufacturing or between different manufacturing sites, with the transfer happening within the same company or between different companies.
A tech transfer involves multiple partners including the sending unit (defined as the organisation a designated process or method is expected to be transferred from) and the receiving unit (defined as the organisation a designated process or method is expected to be transferred to and where it will be executed).
The importance of a dedicated team
As the tech transfer stakes are high, and unforeseen challenges may arise, the creation of a dedicated team able to address multiple areas of the process to be transferred is a good starting point. The tech transfer team may include experts from development, production, quality assurance, regulatory affairs, quality control, and qualification/validation from the sending and receiving units, as well as from the biopharmaceutical company.To operate a smooth tech transfer, the tech transfer team will have to overcome two major challenges:
- Replicate operating procedures with receiving unit equipment
- Complete a full knowledge transfer
Why are CDMO tech transfers so challenging?
Even if two sites use the same equipment and operate the same procedures, it cannot be guaranteed that the processes will behave identically. Even if we can assume that the more similar the equipment train between the two sites, the closer the drug substances & the drug products across sites will be, potentially reducing the regulatory burden of filing a site change, this has to be validated through analytical methods. Those analytical methods also have to be transferred from the sending unit towards the receiving unit.
The importance of ensuring knowledge transfer between staff can also not be over-emphasized. It’s frequent for key details of a process to be unclear, or difficult to find when the receiving unit gets the complete standard operating procedure (SOP).
Key parameters to consider for a tech transfer between different biologics CDMOs
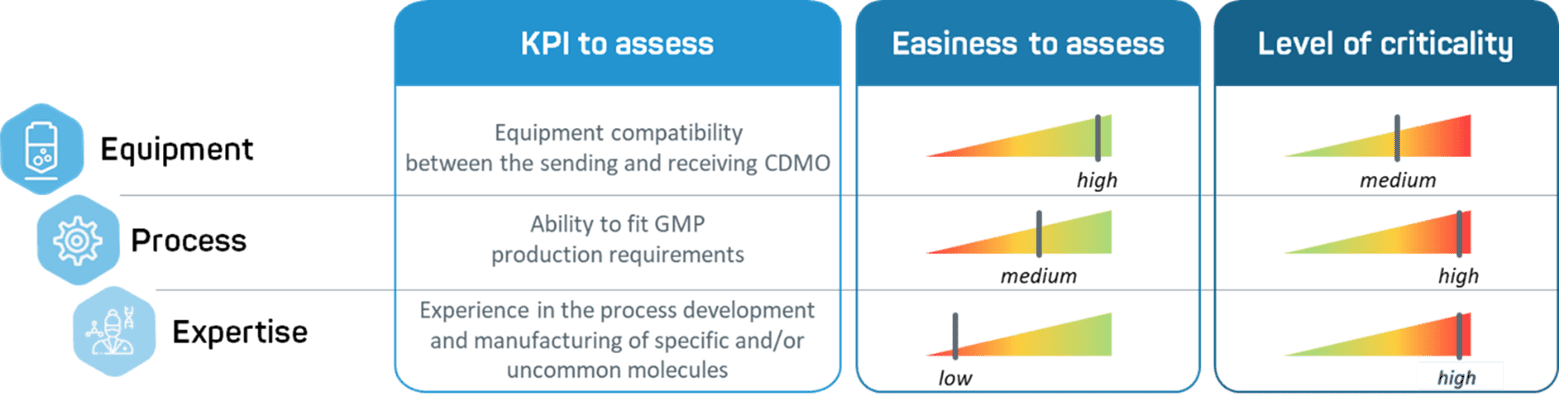
Over the years, we have managed multiple tech transfers, either as a sending or receiving unit. This experience has taught us that expertise, equipment, and process should be considered equally before entering a tech transfer process.
- Equipement. One of the first parameters to examine is the capacities of the receiving site being considered. This is not as simple as seeking the latest technologies – it is important to check equipment compatibility between the sending and receiving facilities.
- Process. The process may have been developed by a partner without a clear understanding of GMP requirements. It is important that the receiving unit determines whether the process can be transferred to its facilities as is, and if it meets GMP requirements. A gap analysis is usually helpful to assess potential process parameter changes during the transfer and to evaluate the impact and risks of such changes.
- Expertise. Transferring to a team with a strong expertise in process development is an asset for a smooth process transfer as the combination of huge scientific expertise and operational skills will allow to identify potential tech transfer risks and/or redesign a process based on minimal information.
Figure 1 – Comparative table of the key parameters to assess while transferring between CDMOs
Process cross-analysis should not be overlooked
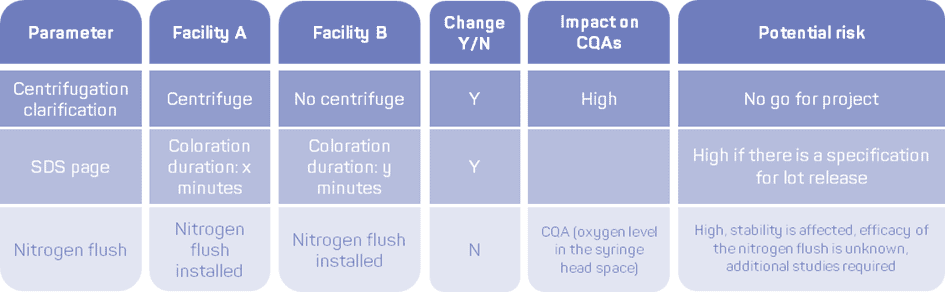
Once the CDMO tech transfer team is established, the next key step is to proceed to a complete cross-analysis of the processes. A detailed and relevant documentation of the processes should be carried out and provided by the transferring site. It will be critical to perform the risk evaluation and gap analysis according to the International Council for Harmonisation (ICH) Q8 guideline. To ensure the tech transfer leads to a robust and reproducible manufacturing process at the receiving facility, critical quality attributes (CQAs) should be identified and then controlled and assessed to achieve the target product profile.
Figure 2 – Examples of differences between the sending and receiving units encountered over the years by GTP Bioways during tech transfers
Although transfer runs have the disadvantage of additional cost and time, their benefits are many including better process understanding and performance, demonstrating product comparability, and verifying CQAs, among others. Transfer runs are also a chance to mitigate risks, solve issues and improve manufacturing instructions.
A few years ago, GTP Bioways was involved in a CDMO tech transfer but only as ‘facilitator’. A biopharmaceutical company had promising results during preclinical phase with a biotherapeutic protein, produced using CHO cells as an expression system. Their CRO partner had recognised expertise in process development, however they did not anticipate all the GMP constraints while developing an efficient process.
During the early process development phase, a GMP manufacturing partner had not been identified. Once the clinical trial partner was identified, a high-yield process was developed. All protocols and required equipment were exchanged. However, the downstream process required high volumes of buffer solution, which meant the receiving CDMO was not able to handle the required quantity of bioprocessing containers in its DSP room. They needed the biopharmaceutical company to downscale the purification process in order for them to be able to handle it in their GMP facility.
GTP Bioways collaborated with the sending and receiving units to adjust the bioprocess to GMP requirements. We had to downscale the bioprocess twice to manage an acceptable quantity of bioprocessing containers in the DSP area. Additionally, we had to implement two chromatography cycles instead of one (in the original bioprocess designed by the CRO). Therefore, we had to investigate the stability of purification intermediaries, develop and validate methods compatible with GMP constraints. Despite a few changes in the process, the project was successfully transferred to GMP manufacturing and passed phase I/II.
Do not underestimate human expertise from both CDMOs
A tech transfer implies having a certain expertise on both sides – the sending unit and the receiving unit. One of the key success factors is the expertise of the CDMO tech transfer teams. Managing successful tech transfers between organizations requires talented people with proficient project management and operational skills combined with scientific expertise.
Communication is the key for knowledge transfer
An additional challenge, often neglected, is clear and effective communication. From the earliest stages of the project until its completion, project management and communication among the project team play a key role. Lack of early and effective coordination between the receiving and sending sites is further complicated by lack of clearly defined roles and responsibilities, lack of communication and poor visibility on timelines, progress, and results. People should be introduced right away, to help build as cohesive a team as possible, so everyone is familiar with who their technical and nontechnical counterpart is and can build bridges among those teams (i.e., upstream, downstream, quality, analytical, etc.).
Alongside bringing together the technical teams of the sending and the receiving units, the project team of the sponsor should dedicate time to ensuring a smooth tech transfer. It can indeed provide guided readings of the important documents to make sure that valuable information is not disclosed too late in the transfer process, avoiding situations where technical solutions need to be found in a short time period, and making it possible to pre-empt possible issues. A strong involvement of the project managers, with dedicated time to prepare meetings, prepare guided batch record readings and provide alerts at early stages is key for a seamless tech transfer. For this, a transfer plan must be set up including a steering committee once a month and working sessions once a week with a defined agenda and appropriate experts invited to move the tech transfer forward.
At GTP Bioways, when we operate a CDMO tech transfer, we always allow a “person in plant” during the optimisation of the development process as well as during manufacturing, to observe and guide in-plant activities. By asking questions and hearing the logic behind all the decisions being made, the receiving team can gain a deeper understanding of the process in real time. Additionally, it gives the transferring facility a chance to jump in when critical or even non-critical decisions need to be made, as well as to help troubleshoot.
Conclusion about tech transfer between biologics CDMO
Drawing on our experience gained through multiple processes transferred, we have built a checklist of the key questions to be answered in light of your reasons for transferring your project and of your project development stage.
- Has the receiving unit got a defined, proven technical transfer plan?
- Are the teams responsive, expert and engaged?
- How easily can open discussions be organised between all involved parties?
- Does the receiving unit demonstrate cost-effective planning and project handling experience?
- Does the receiving partner demonstrate excellence evaluating, assessing, managing, and mitigating risk?
- Does the receiving unit demonstrate stewardship of time, expenses, and schedules?
When you find these attributes and the technical fit to meet your drug’s particular needs, your programme’s success is more likely to follow. Moreover, partners with project managers that demonstrate proficiency in tech transfer and scale up are likely to provide a more solid fit over the long run.