Discover our project management approach as a CDMO. GTP Bioways is an experienced CDMO (contract development and manufacturing organisation) with a recognised expertise in the development and manufacturing of biologics (antibodies, proteins, bioconjugates and other complex biologics) and nanodrugs. Our mission is to partner with our clients to accelerate their product development. To succeed, we want to help you get around these key challenges:
- Developing a scalable and efficient manufacturing process
- Flawlessly handling scale-up, process validation and manufacturing stages
- Achieve quality and regulatory requirements
- Delivering clinical material within timelines with an optimized cost-per-gram
To support your project and help you succeed, GTP Bioways assigns an experienced Project Manager to each project ; whether you’re developing or manufacturing a therapeutic molecule. The project manager is your primary on-site contact, providing effective communication and action follow-up.
GTP Bioways is your partner as you move from laboratory to market. Our CDMO project management team will help you overcome challenges through the entire therapeutics development process.
Our CDMO Project Management approach for biologics and nanodrugs production
GTP Bioways has an efficient and structured set-up, with a dedicated project manager assigned to each project – from process development to fill and finish activities. Each of the individuals on our team is well-versed in more than just their core discipline: they also have a deep understanding of the science behind the projects they oversee. This scientific knowledge allows them to address concerns with direct solutions.
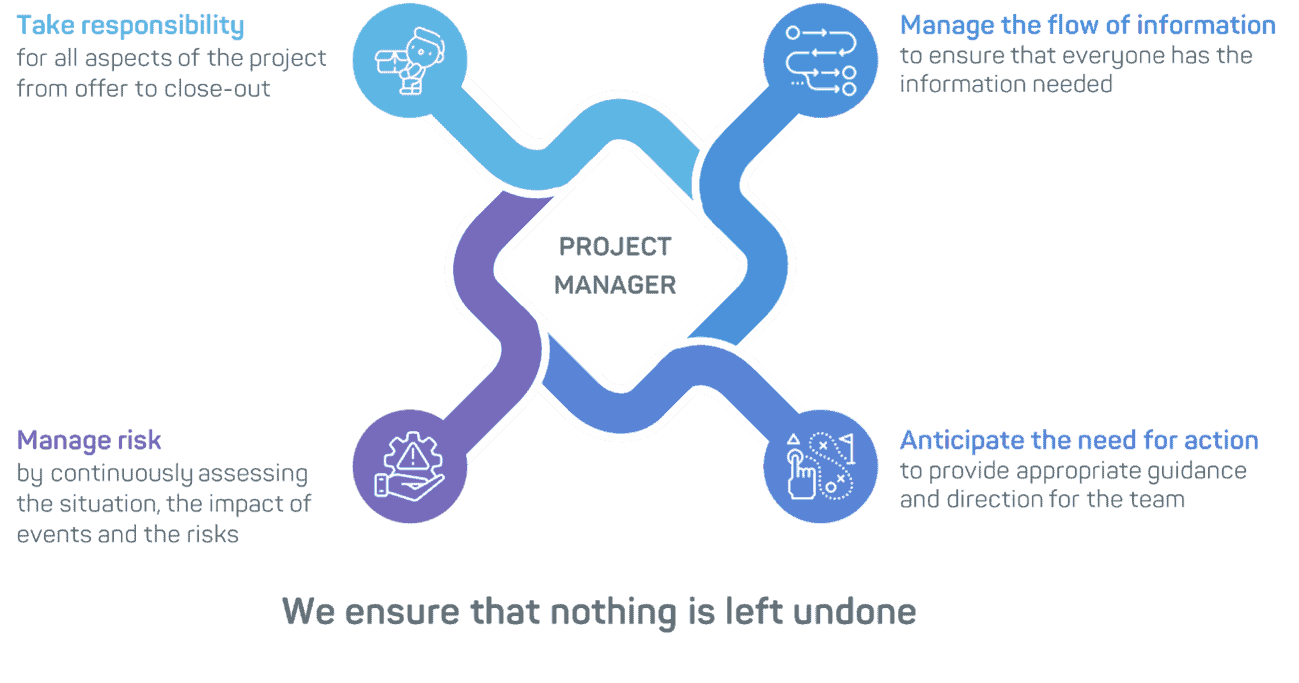
When you work with GTP Bioways, you will deal with a single dedicated project manager able to ‘wear several hats’ and who can discuss your concerns with everyone, from researchers to the financial team and CMC managers. Our Project Management team puts the right set-up in place, plans and coordinates the completion of development and/or manufacturing activities. Among their key activities, the project managers:
- Ensure project objectives are met (quality, costs, deadlines, customer satisfaction) and keep track of project status both in terms of costs and deadlines
- Plan by anticipating changes and risks and identifying appropriate actions, as well as considering the boundaries, budgets, time frames and key stakeholders
- Orchestrate all functions involved in the project (process development, manufacturing, QC and QA…)
Our mission is to provide you with a high level of service and support from beginning to end. We believe your success is our success.
We are a CDMO involved from RFP to delivery and more
The GTP Bioways Project Management team is involved from the initial assessment phase and brings together all project activities throughout the implementation of the project.
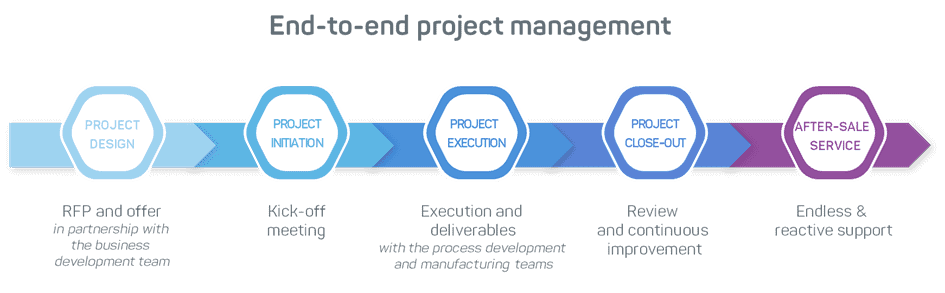
CDMO Project Design
Our project design team is involved from early discussions with our business development team. Backed by their strong scientific knowledge, our team will work with you to design a project that meets your technical constraints. Our expertise enables us to advise you on the most effective way to perform the process development step and further GMP manufacturing of your innovative therapy.
- Appreciate the customer objectives requirements
- Design a workplan that meets technical constraints
- Evaluate the resources required to meet project needs
- Consolidate a proposal and further exchange with the drug developer’s team
Project initiation
- Assemble the team dedicated to the project (pm and experts – Process Development, Analytical Development, Quality Control, Quality Assurance, GMP Manufacturing, HSE …)
- Align on project objectives, organisation with the drug developer’s team
- Define the project governance based on project criticality level
Project execution: from process development to GMP manufacturing
- Lead the project through each technical phase
- Actively manage risk among the project team (project managers, experts) in order to adjust the scope of work based on progress and results
- Closely collaborate with the customer and each partner involved in the project at all stages
- Manage project communication (including regular customer calls & reports)
- Continuous improvement through the project
Project close-out and support
- Formally wrap-up the project with the customer and the business development team
- Extract lessons learned, identify areas for improvement, take actions to improve our approach
- Review customer feedback carefully
- Endless reactive support of the customer
Our mission is to provide outstanding services, solutions and support for our clients. We’re dedicated to creating a positive experience for you every step of the way.