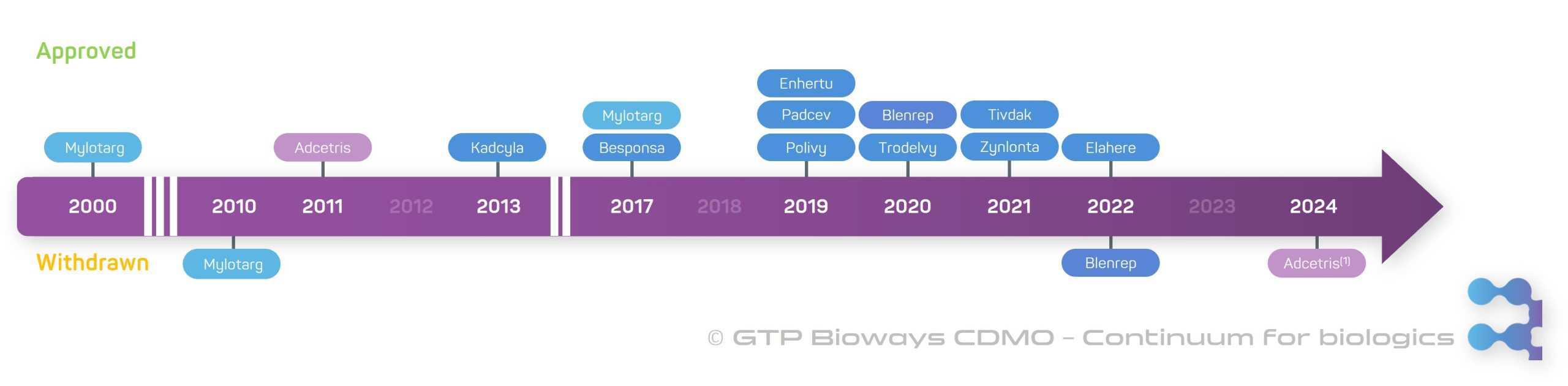
Antibody-drug conjugates (ADCs) stand at the forefront of innovative biotherapeutic agents, representing a rapidly emerging class of bioconjugate products, particularly notable for their potential in cancer treatment. Despite their relatively recent introduction, marked by the approval of the first ADC in 2000 by the FDA (Mylotarg), they have already gained significant attention in the medical community. As of now, 10 ADCs are on the European market. The effectiveness of ADCs is demonstrated across a wide range of tumour types, and it is expected to further expand as advancements in technology lead to the identification of more appropriate target antigens. In this blog post, we will delve into the key characteristics of ADCs and their diverse clinical applications in the field of oncology.
Introduction to Antibody-Drug Conjugates (ADCs)
Antibody-drug conjugates represent a groundbreaking innovation achieved by combining a monoclonal antibody (mAb) with a cytotoxic payload through a linker molecule. Their primary objective is to selectively induce cell death in target cells. Leveraging the specificity of monoclonal antibodies, ADCs precisely target antigens overexpressed in cancer cells, enabling the delivery of potent cytotoxic agents while reducing harm to healthy tissues throughout the body. Essentially, ADCs offer a more precise and effective approach to cancer treatment.
Current Landscape of ADCs in the Market
ADCs represent cutting-edge advancements. For instance, Kadcyla has demonstrated efficacy in treating HER2-positive breast cancer, while Besponsa shows great results for acute lymphoblastic leukaemia (ALL). Over the past 23 years, 12 ADCs have gained approval, with 10 of these approvals occurring in the last 6 years. However, Blenrep, used for multiple myeloma, and Adcetris, used for CD30-positive peripheral T-cell lymphoma, were withdrawn from the market in 2022 and 2024 respectively after failing confirmatory trials.
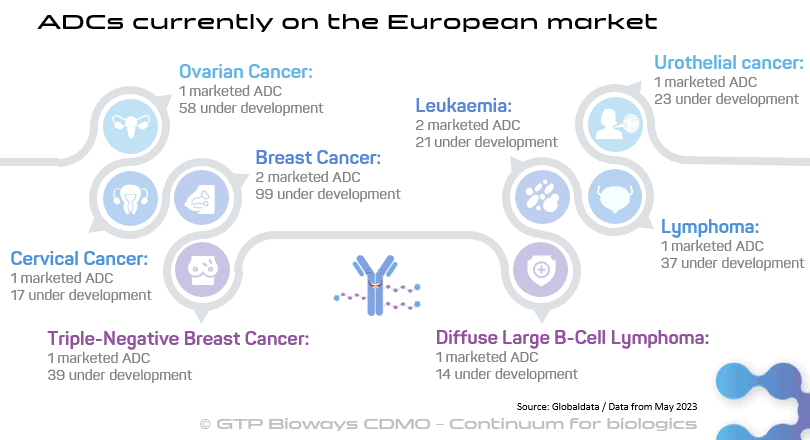
164 ADCs are in various stages of clinical development, with 131 in phase I trials. According to MarketsandMarkets, ADC worldwide sales reached approximately $9,7 billion in 2023 and are projected to soar to $19,8 billion by 2028, indicating a remarkable compound annual growth rate (CAGR) of 15,2%. Although ADCs are currently predominantly used in cancer treatment, research is being conducted exploring their potential applications in a broader spectrum of diseases, including inflammatory conditions and atherosclerosis.
Understanding the Mechanism of Action of Antibody-Drug Conjugates
Selection of antibodies
ADCs use monoclonal antibodies that must exhibit sufficient binding affinity and specificity to effectively recognise and bind to their target antigens on cancer cells. This enables the precise delivery of the cytotoxic payload to cancer cells while preserving healthy cells from harm. Each ADC is customised to align with the antigen profile of the cancer type under treatment.
Selection of the linker
Once the ADC binds to the cancer cell’s surface, the choice of linker becomes crucial for its function. Cleavable linkers, for instance, allow the payload to detach from the antibody at the tumour site via hydrolysis or enzymatic cleavage. Currently, they constitute 80% of all ADCs. This predominance owes to the bystander effect, where the released payload diffuses from the disintegrated ADC cells to neighbouring cells within the tumour microenvironment. While cleavable linkers are more effective, they tend to induce higher toxicity compared to non-cleavable ones. Non-cleavable linkers facilitate antigen-specific internalisation by cancer cells, followed by complete lysosomal degradation of the antibody. This mechanism grants ADCs longer plasma half-lives, reduces off-target toxicity, and widens their therapeutic window.
Activation of Cytotoxic Action Resulting in Cancer Cell Death
ADCs typically integrate cytotoxic compounds that are too potent for independent use as anticancer drugs. Even at low concentrations, these cytotoxic agents demonstrate remarkable effectiveness. They disrupt vital cellular processes, such as DNA replication or microtubule assembly, hindering the cancer cell’s capacity to proliferate and survive, ultimately leading to cancer cell death.
ADCs offer a promising alternative to chemotherapy, providing a precise targeting mechanism and mitigating the adverse toxic effects associated with traditional chemotherapy.
Addressing Challenges Faced by Antibody-Drug Conjugates
Potential cancer resistance to ADCs
Cancer cells can gradually develop mechanisms to resist ADCs, reducing their effectiveness over time. This resistance may arise from various factors, including altered target cell surface expression or gene mutation, resistance to the ADC payload, failure in internalisation processes, impaired lysosomal function, or activation of drug-efflux pumps. Strategies to counteract this resistance include developing ADCs with alternative linkers and payloads, employing combined therapies that target complementary pathways, and enhancing immune-mediated killing of tumour cells by integrating immune checkpoint inhibitors into ADC treatment regimens.
ADC facing tumour heterogeneity
Cancer cells may exhibit diverse antigen expression levels, potentially reducing the efficacy of ADCs. To address this limitation, strategies like multispecific and bispecific antibody-drug conjugates, which incorporate multiple targeting domains, or combining ADCs with other therapies can broaden the spectrum of targetable antigens and enhance tumour penetration.
Off-target toxicities risk for ADC
A significant challenge associated with ADCs is the risk of off-target toxicities, especially with those using cleavable linkers. This can result in haematotoxicity, hepatotoxicity, and gastrointestinal reactions. Cleavable linkers often undergo hydrolysis in the bloodstream at a notable rate, causing the payload to be released prematurely in the extra-tumoral compartments, leading to unintended cytotoxicity. One approach to mitigate this toxicity is to enhance the stability of ADCs. Highly loaded ADCs are often unstable in plasma and exhibit high rates of non-specific uptake in the liver. This challenge can be addressed by reducing the Drug to Antibody ratio (DAR) or modifying the linker to decrease ADC hydrophobicity and toxicity.
Expert process development to minimise aggregation of ADCs
ADCs are prone to aggregation, particularly during the initial stages of development. Aggregation can induce structural alterations, potentially compromising the conjugate’s ability to effectively bind to the target antigen. One way to overcome this challenge is to prevent aggregation through immobilisation of the antibodies on a solid-phase support, such as resin, and carry-out the conjugation of the payload-linker while the antibodies are bound to that support.
Expensive cost of antibody-drug conjugates
A significant barrier to the widespread adoption of ADCs as a viable therapeutic option is their elevated cost. This cost encompasses expenses related to development, manufacturing, and administration, thereby restricting accessibility, particularly in certain regions or healthcare systems.
Efforts to address these challenges are essential to further advancing the clinical use and accessibility of ADCs in the realm of modern medicine.
Only time will tell if antibody-drug conjugates are the future of cancer treatment
Antibody-Drug Conjugates stand at the forefront of innovative biotherapeutic solutions, showcasing immense potential in cancer treatment by offering targeted therapy and enabling precise cell death. This sets ADCs apart from conventional chemotherapy, reducing collateral damage to healthy tissues and minimising adverse effects. ADCs provide hope for patients seeking alternatives to traditional chemotherapy.
Despite their relatively recent introduction, ADCs have garnered significant attention within the medical community. 10 ADCs are currently on the market and numerous others in various stages of clinical development. Even if their effectiveness spans across a wide range of tumour types, scientists are actively exploring their potential applications across various therapeutic areas, extending beyond oncology, and thereby expanding bioconjugates therapeutic potential.
Please note that all drug name mentioned are registered trademarks.