What you will read about: Conclusions of our freedom to operate studies on T7 promoter system.
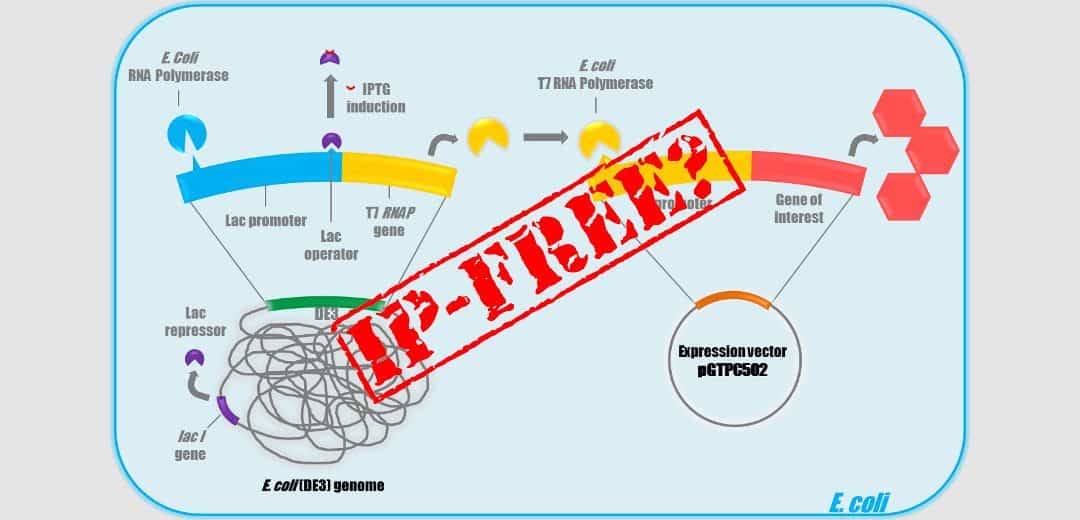
T7 promoter system is the most widely used promoter for Bioproduction in Escherichia coli. Classically this system is separated in two parts : an E. coli expression vector that carries a T7 promoter and an E. coli production strain DE3 that contain a lambda lysogen with an inducible T7/ RNA Polymerase (RNAP) gene on its chromosome.
This system was described for the first time in 1986 by Studier in the article « Use of bacteriophase T7 RNA polymerase to direct selective high-level expression of cloned genes ». Many patents protect the T7 system but only one could be troublesome : the patent US5,693,489 « Cloning and expression of the gene for bacteriophage T7 RNA polymerase ». This patent has a filing date of June 14th, 1994 and was issued in December 2nd, 1997. It has been filed under priority of the US patent application No. 595,016 filed in March 30th, 1984. The inventors are F. William Studier, Parichehre Davanloo, Barbara A. Moffatt and John J. Dunn.
This patent claims : a method for controlling the expression of a target gene, comprising :
a) introducing into an E. coli host cell containing a gene encoding an RNA polymerase from a T7 bacteriophage, a target gene operatively linked to a promoter recognizable by the RNA polymerase from the T7 bacteriophage ; and
b) maintaining the host cell under conditions appropriate for expression of the target gene by the RNA polymerase from the T7 bacteriophage.
Furthermore, in the patent description, it is mentioned that T7 RNA polymerase is also used in a system for selective, high-level synthesis of RNAs and proteins in suitable host cells.
So, our recent “Freedom to operate” studies, performed on our E. coli vectors and strains, showed that the T7 system appears now definitely free of any rights since the last patent on the system that protected the use in the United States expired on December 2014. This patent protected also BL21 (DE3), BLR (DE3), HMS174 (DE3), JM109 (DE3), and OrigamiB(DE3) E. coli strains. So in principle, one could imagine that it is now possible to freely use these strains for commercial production. Unfortunately, that is not so easy since you may read on the Novagen site : « This product is covered under license from Brookhaven National Labs. Commercial entities need to obtain a research use license prior to purchase. Please contact your licensing department to confirm that your company already holds a research use license ».
In text, this exploitation issue seems therefore more connected to a contractual aspect related to the strains origin than to a problem of intellectual property. According to us, a smart way to avoid jeopardizing a bio-production project, would be to construct de novo E. coli strains T7/RNAP but without the DE3 prophage. This can be done easily by companies like BGene. The benefit would be twofold : no more contractual issue and spontaneous lysis due to DE3 prophage excision. Although this strategy involves a more detailed characterization of the new production strain, inherent timescale and additional cost do not question the relevance of this approach.
Once again, do not hesitate to react : the debate is open.