Bioconjugates manufacturing
GTP Bioways is an expert CDMO for the process development and manufacturing of antibody-drug conjugates.
Comprehensive CDMO services for antibody drug conjugates
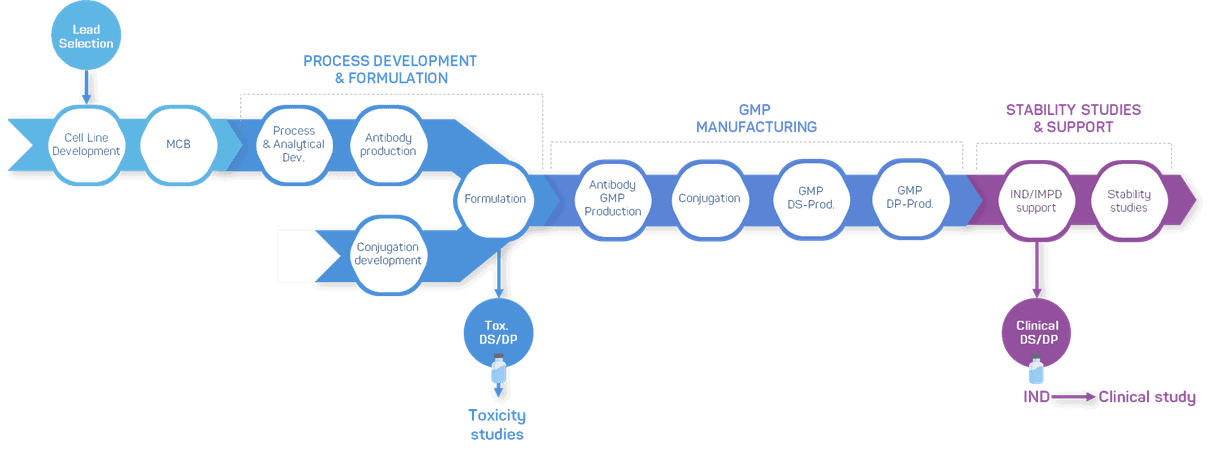
GTP Bioways’ activities cover the full development cycle of your bioconjugate, from supporting cell line and conjugation development to cGMP manufacturing.

Flexible ADC manufacturing services
GTP Bioways has the expertise and capabilities to develop ADC production processes for a wide range of conjugation technologies.
Our facilities are fully equipped to perform ADC conjugation and manufacture up to kilograms (depending of the coupling concentration). We have the expertise and equipment to deliver both non-GMP material for your preclinical efficacy and toxicology studies, and GMP material.
In addition, our team can support the process development and manufacturing of your antibody thanks to our in-house capacities.
Cutting-edge manufacturing facilities for ADCs
Our cGMP production suite is sized for up to hundred-liter scale production of antibody-drug conjugate.
Our facilities are equipped with:
- PSL isolator for cytotoxic handling
- Stainless steel reactor for conjugation
- Single-use purification equipment (chromatography and TFF)
Along with well-established controls for handling cytotoxic materials, we have implemented a quality system and documentation in compliance with CFR guidelines.


A robust formulation development of your antibody-drug conjugate
As part of our process development package, our team will handle the formulation of your drug substance.
We use Design of Experiment (DoE) principles to optimise the formulation of your molecule whilst mitigating risk.
After identifying which excipients and conditions allow for maximum product stability, we test them in parallel through systematic, real-time accelerated studies.
A dedicated analytical team for characterization of your ADC
Complementary to our cGMP manufacturing capacities, GTP Bioways has a dedicated cGMP analytical lab equipped with cutting-edge instruments and knowledgeable analytical chemists, to support process development, batch release and stability assays of drug substances and drug products.
Our specific analytical package for ADCs includes a range of methods among which:

- Drug Antibody Ratio (DAR) and quantification of residual unconjugated drug
- Fragment profile, Size exclusion chromatography
- Micro-Flow Imaging
- Cytotoxicity
- Residual solvent, if any solvent is used during the manufacturing process
- Mass spectrometry, used for characterisation and batch release
Our analytical team has the expertise to handle method transfer, method development and validation to have the most robust process to ensure the success of the cGMP manufacturing of your drug product.

An exclusive aseptic fill & finish solution
GTP Bioways is one of the few CDMOs covering the whole value chain thanks to our in-house aseptic fill & finish. With our exclusive semi-automated filling line and its single-use isolators, we offer flexible manufacturing of drug products including highly potent drugs.
When a lyophilized formulation of the ADC is required, we work with a long-standing partner to offer appropriate lyophilization and fill and finish services.