Custom Immunoassay and ELISA Development for Biologics
At Olon, we know that robust immunoassays are essential to ensure the safety, efficacy, and regulatory approval of biologics. With more than 20 years of experience, our dedicated team develops and validates custom immunoassays and ELISAs tailored to your molecule, even when no commercial kit is available.
Expertise in Immunoassay Development
Our scientists design and optimize assays for a wide variety of applications:
Host Cell Protein (HCP) detection to secure bioprocess safety
Contaminant and impurity testing (e.g., trypsin, Benzonase, dsRNA)
Potency testing through bioassays and ELISA-based quantification
Cleaning validation for GMP processes
Drug substance batch release testing
We have successfully developed immunoassays for monoclonal antibodies, cytokines, ADCs, and other complex biologics, even in challenging matrices such as harvest fluids or formulated buffers.
Our approach for custom immunoassays and ELISA
Every assay is developed in the required format and according to the agreed conditions of your program. Once designed, the assay is systematically optimized for accuracy and sensitivity.
A crucial step is testing matrix effects to ensure reliable performance in real samples:
The measuring matrix is carefully characterized.
Accuracy is assessed by spiking experiments.
Precision is verified through repeat testing.
We perform pre-validation according to ICH guidelines, ensuring that each assay meets defined accuracy and precision acceptance criteria. Following this, we seek customer approval before moving into pilot production.
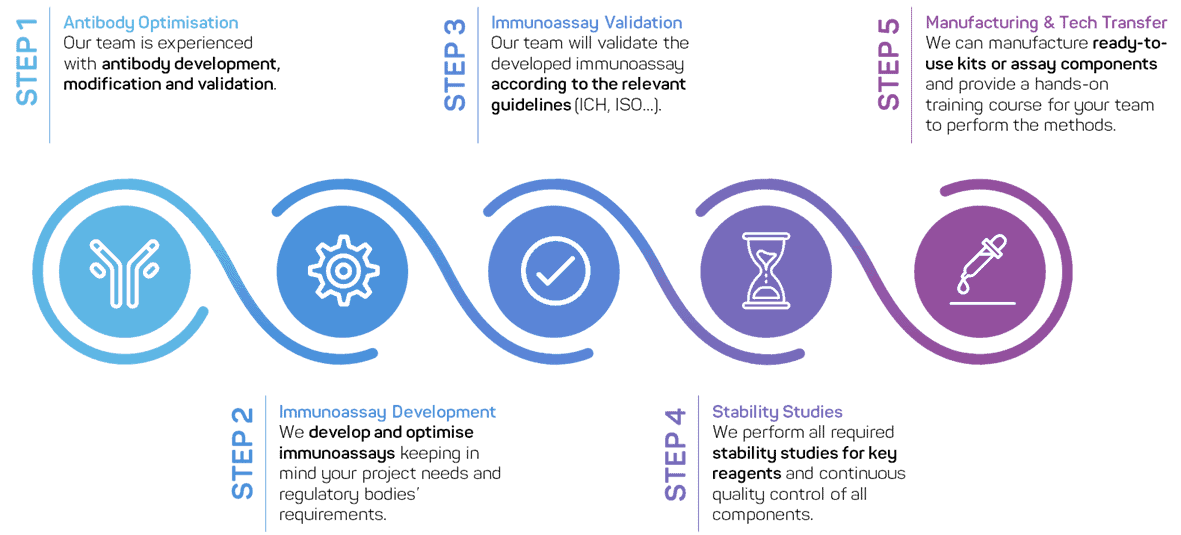
If the method is to be delivered as a kit format, we conduct real-time stability testing to guarantee consistent performance. We can supply:
Fully developed and optimized reagents for straightforward in-lab use
Dozens or hundreds of ready-to-use kits, including pre-coated plates and all necessary reagents
This ensures your immunoassay is not only scientifically robust, but also practical, scalable, and regulatory-ready.
Example Applications
Our custom immunoassays and ELISAs are used across a wide range of biologics programs. Typical applications include:
HCP assays for biologics and biosimilars, ensuring patient safety and regulatory compliance
Potency assays for monoclonal antibodies, fusion proteins, and ADCs
Comparability studies to demonstrate biosimilarity or process changes
Cleaning validation assays to verify removal of process contaminants
Impurity assays (e.g., Benzonase, trypsin, dsRNA) to support regulatory submissions
Release testing for clinical and commercial GMP batches
By tailoring each assay to your molecule and process, we ensure that the data generated is reliable, compliant, and decision-ready.
Webinar
HCP Risk Management
Vincent Rivera, founder and director of the BU Immuno is sharing his great experience regarding HCP (host cell proteins) and biocontaminants risk management.

Integrated Analytical Services
Immunoassay development at Olon is embedded within a comprehensive analytical platform, enabling us to provide consistent, end-to-end support for your biologics:
Method development, optimization, and validation across a broad spectrum of assays
HCP risk management strategies, from platform ELISA to fully custom assays
Stability and formulation testing, including forced degradation and comparability studies
Mass spectrometry, 2D DIGE, and orthogonal techniques to complement ELISA data
Regulatory-compliant documentation aligned with ICH, EMA, and FDA requirements
Analytical transfer and training, supporting routine QC implementation at your site
By combining cutting-edge technology with regulatory expertise, we deliver analytical solutions that not only generate robust data, but also strengthen your CMC package and accelerate regulatory approval.
