Our partner PROTEODYNAMICS, a glycan analysis and protein characterization expert, reviews the relevant methods to characterise the glycosylation of biotherapeutic candidates.
Post-translational modifications, such as glycosylation, are important factors defining activity, efficacy and functionality of proteins in higher eukaryotes. The ability to reproduce post-translational modifications in an appropriate fashion has to be considered in bioprocess development, in order to guarantee therapeutic efficiency and regulatory acceptability of the recombinant product. There are two main types of protein glycosylations: N-glycosylation, in which the glycan is attached to an asparagine residue (Asn) present in the consensus sequon Asn-X-Ser/Thr (where X can be any amino acid except proline) and O-glycosylation, in which the glycan is attached to a serine (Ser) or a threonine residue (Thr).
According to pharmaceutical guidelines, it is important to answer various questions concerning glycosylation during bioprocess development, such as: is the protein of interest glycosylated? Which glycan structures are present and what are their proportions in the protein? Where are glycans linked to the protein and what are the levels of occupation and the types of glycans bound to different glycosylation sites? Does glycosylation of the protein stay the same during process development and scale-up and is it consistent from batch to batch? Indeed, it is known that the selected expression system as well as bioreactor conditions (nutrient levels, pH, oxygen content or for example cell density) can influence the glycosylation of the expressed protein.
Several analytical methods are available to help elucidate the glycosylation of recombinant proteins starting at the level of the intact protein and going down to detailed analysis of glycan profiles and structures and to analysis of different glycosylation sites of the protein.
Determination of the total mass of the intact glycoprotein by mass spectrometry will allow to observe the variation of protein mass due to the heterogeneity of the attached glycan moieties. Depending on the mass spectrometer used, this analysis will determine an average mass (MALDI-TOF MS) or an accurate mass (HR/AM MS). But even this accurate analysis does not allow to avoid a more detailed structural one.
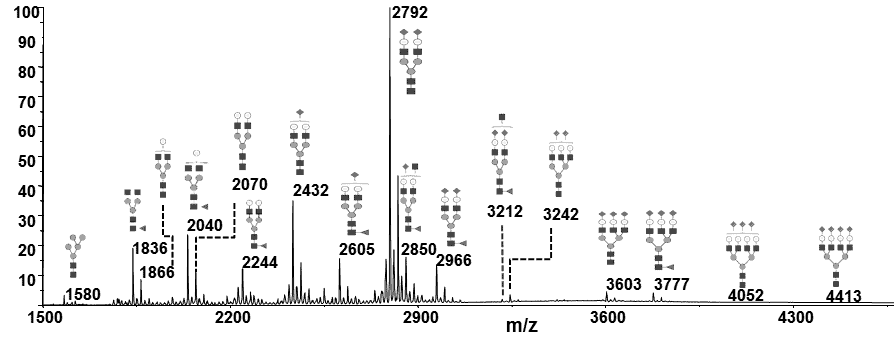
The most common manner to perform glycosylation analysis during bioprocess development involves the glycoprofiling of liberated intact glycans from the entire glycoprotein. Intact glycans are thereby released either enzymatically (N-glycans) or chemically (O-glycans). PROTEODYNAMICS recommends analysing glycan profiles early on when the first research batches of the protein are available. Particular attention should be paid to which structures are present and their antennary profiles should be determined, which means the degree of mannosylation, fucosylation, galactosylation and sialylation of the glycoprotein should be measured. This allows to observe the variability of the profile during process development and to determine a reference profile to compare glycosylation between different batches produced. Glycan profiling can help select the best cell clone producing a recombinant protein matching the required or desired glycosylation profile. In addition, the presence of non-desired glycan structures carrying for example immunogenic sugars or epitopes can be controlled.
Glycan profiling will determine the relative intensity (%) of individual glycans present in the overall glycan profile of a glycoprotein. Several complementary analytical approaches are available. In general, N-glycans are released by deglycosylating enzymes such as PNGase F, isolated, chemically modified by permethylation or fluorescence labeling and analysed through different techniques such as hydrophilic interaction liquid chromatography (HILIC), capillary electrophoresis (HPCE) or by mass spectrometry approaches like electrospray ionization mass spectrometry (ESI-MS) or time-of-flight mass spectrometry (MALDI-TOF MS). To analyse O-glycosylation, O-glycans are released chemically by b-elimination prior to their chemical modification and analysis through the above-mentioned techniques. All these provide reproducible results, but none of them allows to generate “absolute results”. PROTEODYNAMICS recommends analysing a reference protein in parallel if available, or defining the protein from a particular production batch as a reference. The glycan profiling is a key control to validate the production of the glycoprotein.
Finally, occupancy and specific glycosylation of individual glycosylation sites should be analysed with the first production batch to complete the characterisation of the protein. After digestion of the glycoprotein by an appropriate protease such as trypsin to obtain peptides, a specific separation of glycopeptides followed by mass spectrometry analysis allows a detailed characterisation of the glycans bound to each glycosylation site. This analysis provides qualitative answers to the occupancy of glycosylation sites and the O-or N-glycans bound to specific glycopeptides can be compared to the results of O- and N-glycan profiling.
If required and in addition to the above-mentioned approaches, monosaccharide composition can be determined by HPAEC PAD or HPLC, sialic acid analysis can be performed by HPAEC PAD, DMB labeling and HPLC analysis and lectin based analyses can be used to determine for example the specific type of sialic acid linkage.
In conclusion, it is only through combining methods that you will obtain reliable information on the glycan profile of your protein or antibody. The experts at PROTEODYNAMICS are always at hand to give advice on what methods may be most appropriate for you depending on what stage you’re at in your programme development and the question you want to answer. So if you are looking for support to characterise the glycosylation of your glycoprotein, do not hesitate to contact them!