End-to-End ADC Manufacturing and Bioconjugation Services
At Olon, we deliver comprehensive ADC manufacturing services to accelerate the development of antibody-drug conjugates from preclinical development through clinical supply.
One of our facility is dedicated to mAb production and GMP bioconjugation, while one of our Italian facility provide OEB6-certified facilities for payload and linker manufacturing. By working hand in hand, we ensure that every critical component of an ADC — antibody, payload, linker, conjugation, and analytics — is managed seamlessly within the Olon network.

Integrated ADC CDMO Capabilities
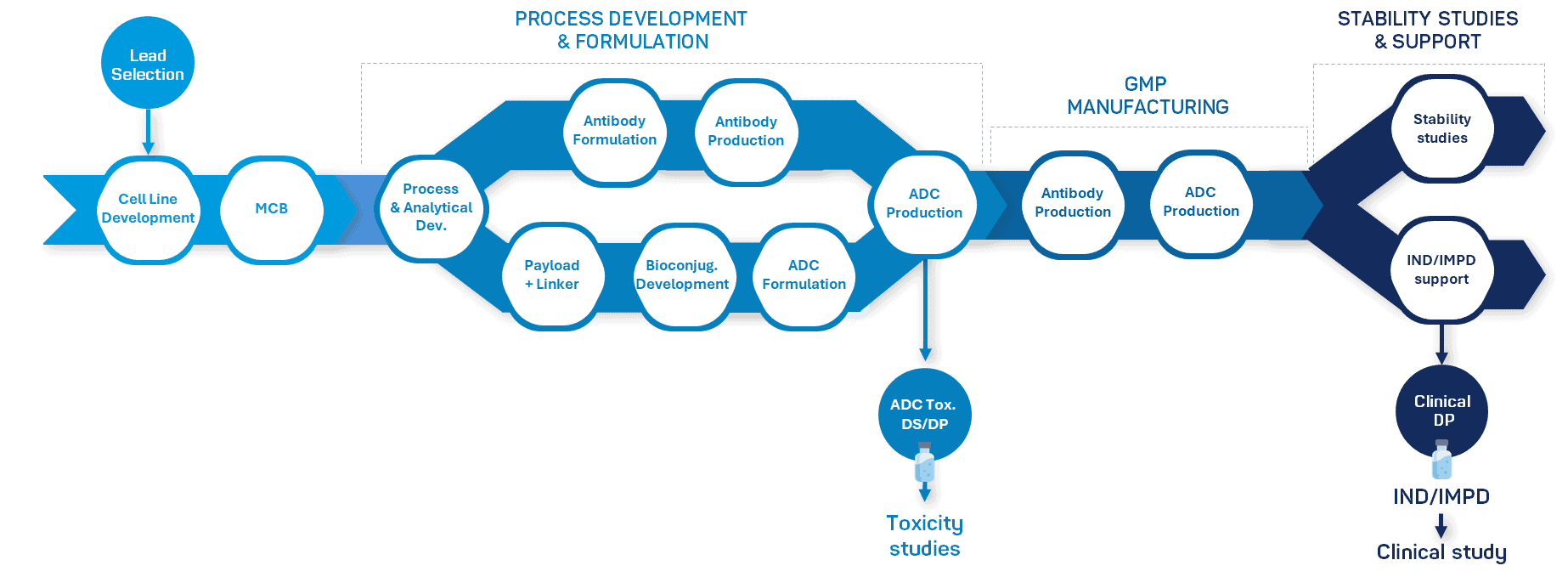
At Olon, we understand that developing antibody-drug conjugates requires more than just expertise in one discipline — it takes seamless integration across the entire ADC value chain. That’s why we’ve built a platform that brings together all the critical elements under one roof, ensuring speed, consistency, and regulatory confidence.
Antibody supply – Mammalian GMP production (10 L to 2 000 L)
Payload & linker production – OEB6-certified facilities for ultra-potent HPAPIs (OEL down to 10 ng/m³)
Bioconjugation services – GMP ADC conjugation in France, with multiple conjugation strategies and DAR control
Analytical expertise – 60+ scientists supporting development, release, and stability testing
Technical Depth in Bioconjugation
Our French ADC suites are designed with high-containment and single-use systems, offering safety, flexibility, and consistency.
Site-specific antibody conjugations for batch-to-batch consistency, high payload homogeneity, and optimal thermal stability
Expertise across a wide range of linker chemistries (maleimide, engineered cysteine, enzymatic)
Capability to handle diverse payload classes (small molecules, toxins, radionuclides)
High-containment chromatography and TFF systems for effective purification
In-process control for DAR determination, residual linker, and free payload quantification

Streamline your ADC production with our comprehensive development & manufacturing services
With our unmatched expertise and capabilities, we seamlessly cover every stage of your antibody drug conjugate project, from antibody intermediate production to bioconjugation and GMP manufacturing of drug substance and drug product.
Analytical Excellence for ADCs
Robust analytical characterization is critical to ensure the safety, efficacy, and regulatory approval of antibody-drug conjugates. At Olon, we provide a full suite of CMC-driven analytical services, all performed in GLP-compliant laboratories by a team of highly skilled scientists.
Our capabilities include:
Drug–Antibody Ratio (DAR) & Residual Free Drug – precise quantification of conjugated payload and unbound toxin
Fragment Profile by Size-Exclusion Chromatography (SEC) – aggregation and fragmentation analysis
Micro-Flow Imaging – subvisible particle counting and visualization

- Cytotoxicity Bioassays – potency testing to validate activity post-conjugation
Residual Solvent Analysis (GC–MS) – trace detection in line with ICH guidelines
- Mass Spectrometry (intact & peptide-level) – detailed characterization, DAR confirmation, and batch-release data
Our analytical scientists deliver actionable data on ADC purity, potency, and safety through comprehensive method development, transfer, and validation. This ensures a robust workflow that de-risks development, streamlines regulatory review, and underpins the successful GMP manufacturing of ADCs.
Regulatory Support & Compliance
With decades of experience in high-potency GMP manufacturing, Olon offers regulatory expertise that de-risks and accelerates your ADC program:
Integrated CMC documentation for IND/IMPD submissions
Audit-ready facilities and validated processes
Experienced regulatory affairs team aligned with EMA/FDA requirements
Successful track record in supporting ADC clinical batches
FAQ on GMP ADC Manufacturing & Bioconjugation Services
What are the key benefits of antibody-drug conjugates in therapeutic development?
Antibody-drug conjugates (ADCs) harness the targeting precision of monoclonal antibodies to deliver highly potent cytotoxic payloads directly to tumor cells, significantly reducing off-target effects and systemic toxicity. This targeted approach often translates into higher therapeutic indices and improved patient tolerability. At GTP Bioways, we support ADC innovation from early linker design through full cGMP manufacturing, ensuring each conjugate achieves optimal stability, efficacy, and safety for clinical success.
What bioconjugation and GMP ADC manufacturing services does Olon offer?
We provide end-to-end ADC services, including site-specific conjugation using maleimide, engineered cysteine, or enzymatic linkers; scale-up protocols from milligram R&D runs to 100 L clinical batches; OEB6 isolator technology for safe HPAPI/eHPAPI handling; both stainless-steel and single-use downstream purification (chromatography & TFF); and comprehensive documentation per 21 CFR Part 210/211. Each project benefits from tailored process optimization, tech-transfer support, and real-time project management to keep timelines on track.
How does Olon ensure GMP compliance and high-quality ADC manufacturing?
Our cGMP production suites are ISO-certified and audited to FDA, EMA & PMDA standards. We enforce rigorous SOPs, FMEA-based risk assessments, environmental monitoring, CAPA workflows and change control—delivering audit-ready batch records and consistent product quality.
What analytical and CMC support does Olon provide for ADCs?
In our GLP-compliant labs, we offer a comprehensive CMC package: DAR and residual free-drug quantification; fragment profiling by SEC; subvisible particle analysis via micro-flow imaging; functional cytotoxicity bioassays; residual solvent testing by GC-MS; and intact-/peptide-level mass spectrometry for detailed characterization and batch release. We also specialize in analytical method development, transfer, and validation, ensuring your regulatory submissions are backed by rigorous data.
How do you determine and control Drug-Antibody Ratio (DAR) and residual free drug?
DAR is initially measured by hydrophobic interaction chromatography (HIC) and then confirmed with high-resolution intact mass spectrometry. We fine-tune conjugation parameters—such as antibody reduction level, linker stoichiometry, and reaction time—in our controlled bioreactor environment to achieve your target DAR. Post-conjugation, we run batch-release assays to quantify any unbound payload, ensuring free-drug levels remain below critical safety thresholds.
What containment technologies are used for cytotoxic payloads in ADC production?
We utilize OEB6 isolator technology—the highest current containment standard—to protect operators and the environment when handling HPAPIs and eHPAPIs. Our isolators feature negative-pressure cascade control, HEPA-filtered exhaust, and glove-box interfaces, eliminating cross-contamination risks and ensuring payload integrity throughout conjugation steps.
Can Olon scale and customize ADC manufacturing processes?
Absolutely. Our flexible platform spans 1 L–100 L conjugation reactors and 10 L–200 L single-use downstream systems, with validated scale-up into 200 L–2 000 L GMP suites. We customize linkers, payload chemistry, and process parameters based on your molecule’s properties, and provide full tech-transfer packages—including batch records, SOPs, and on-site support—to ensure a smooth transition to larger scales.
How does ADC process development integrate with clinical trial phases?
From preclinical proof-of-concept through Phase I/II/III, we align conjugation and manufacturing milestones with your clinical timelines. We supply GMP-grade clinical trial material, conduct stability studies under ICH conditions, and generate CMC documentation to support IND, CTA, and BLA filings. Our collaborative approach ensures that each regulatory submission is backed by robust data and that manufacturing readiness keeps pace with your trial progression.


