Why integration of continuous chromatography could help the biopharma industry overcome the challenge of increasing capacities while decreasing manufacturing costs.
In this article, Vincent Monchois, Director of Strategic Projects at Novasep, our CMO partner, explains why integration of continuous chromatography could help the biopharma industry overcome the challenges of increasing capacities and decreasing manufacturing costs.
Producing active pharmaceutical ingredients requires the use of quality and safety assurance processes while keeping control over manufacturing costs – which are put under further pressure by increased demand and the emergence of biosimilars. It’s possible to scale up production without significantly increasing specific costs: thanks to cell line engineering, the optimisation of the culture media composition and the development of new bioreactor culture methods (i.e. continuous high-density culture), specific productivity has increased. But scaling up purification processes still comes with an at least linear increase in costs. The development of technological solutions for the purification of biomolecules therefore remains a key challenge when it comes to the growth of the biopharmaceutical market.
Challenges linked to the separation of active pharmaceutical ingredients: focus on chromatography
When manufacturing an active biopharmaceutical ingredient, the separation process is key to achieving the expected specifications and guarantee the quality and activity of the product. Chromatography is a key step within this process. It allows to separate the molecule of interest from a mixture due to the differences in behaviour it exhibits within a mobile phase (i.e. liquid phase in bioseparation processes) when passing through stationary phase (i.e. a resin packed in a column). Several steps are required in a chromatography cycle in order to purify this protein of interest. For example, in the purification of monoclonal antibodies (mAbs), their capture is performed by affinity on a protein A resin using a 5-step cycle: sample loading, washing the column to remove unretained species, elution of the molecule of interest, regeneration and equilibration. How the process is scaled depends on the capacity and selectivity of the stationary phase, the number of steps within a cycle, and the duration of a cycle. The main limitation lies in the fact that capacity varies inversely to speed. Process scaling is conventionally carried out through increasing the volume of resin while multiplying the number of cycles (batch mode). Costs therefore increase at least proportionately, when taking into account increased buffer volumes and equipment size (columns and pumping equipment).
Continuous chromatography: a look back at 50 years of industry change
In order to address this economic issue, continuous chromatography processes have been implemented on an industrial scale to replace batch mode chromatography. Continuous chromatography thus appeared in the 1960s oil industry in the form of the simulated moving bed (SMB) process. At the time, the challenge was to produce high-purity p-Xylene on an industrial scale by separating it from its isomers. In the 1970s, the food industry applied this approach to the separation of sugarcane molasses and beet molasses by developing the less rigid and more productive SSMB (Sequential Simulated Moving Bed) process. The 1990s marked the arrival of technology in the pharmaceutical industry as an answer to the increasing number of chiral molecules. Closed-loop high performance multi-column continuous chromatography processes were used in the production of commercial batches of chiral molecules such as Keppra (UCB) or Zoloft (Pfizer).
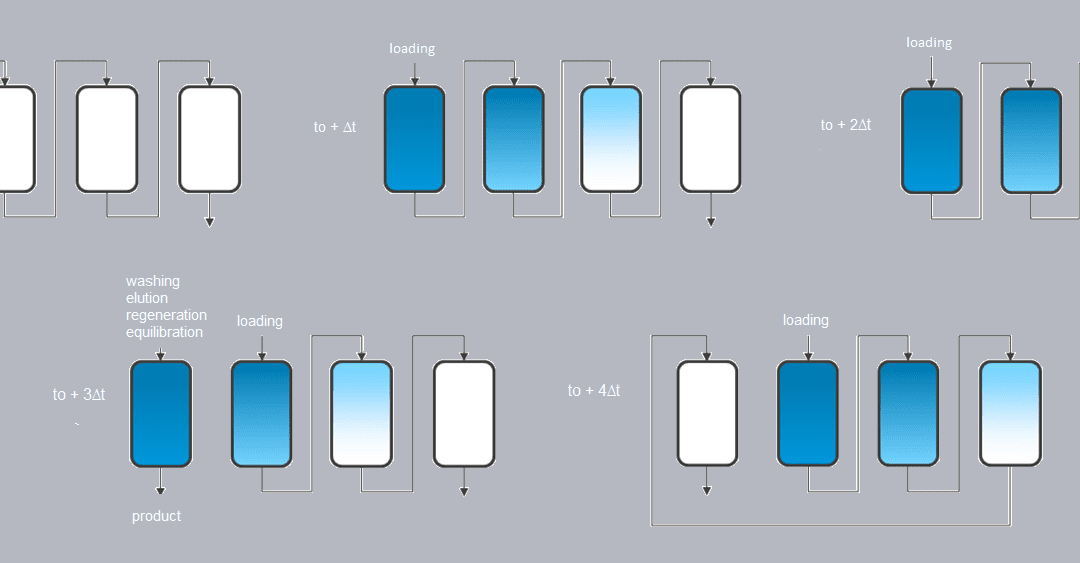
Application of continuous chromatography to the pharmaceutical industry: the example of SMCC technology
The role of continuous chromatography to face the economic challenges stemming from the rise of the biomolecules market, especially mAbs, seems hard to deny. Sequential multicolumn chromatography, or SMCC, is for example an alternative to traditional batch capture processes, as in the case of the capture of a mAb on a protein A resin. SMCC is an open-loop continuous chromatography process allowing the separation of different compounds through the use of different columns (usually 2 to 6). It works as follows (Figure 1):
– The first column is loaded (for example with the culture medium containing the antibody), the columns being connected together. When approaching maximum capacity of the first column, capture moves on to the following columns. At maximum capacity, a washing stage is introduced in order to move all product from the interstitial volume onto the next column.
– The first column is then disconnected and the cycle continues while loading continues from the next column.
– At the end of the cycle, the first column is reconnected and the process starts again from the second column.
– This sequence is repeated to get to a permanent pattern where the load is continuously injected.
This technique makes the most of resin capacity without any loss of product of interest. By making it possible to reach total capacity (or static binding capacity), this technology allows higher throughput rates than batch chromatography. At an industrial scale, with SMCC, thanks to increased capacity, resin and buffer volumes, and therefore equipment size, can be reduced. This leads to a 2- to 4-fold gain (Figure 2). Increased throughput rates mean productivity (kg of product / kg stationary phase / 24h) is also considerably improved.
Conclusion
In order to reach expected production costs for separation processes, the biopharmaceutical industry must integrate continuous chromatography techniques, such as SMCC. The resulting increase in performance also leads to optimised capacity, allowing for more flexibility in a changing market.