Comprehensive Bioconjugate Process Development for ADCs and Novel Conjugates
At Olon, we specialize in bioconjugate process development to help biotech and pharma innovators transform complex conjugates into safe, scalable, and regulatory-ready products. With over a decade of experience in conjugation technologies, and 40+ years of high-potent API manufacturing across the Olon network, we deliver solutions that span from early feasibility through to clinical and GMP production.

Broad Capabilities Across Bioconjugates
At Olon, our team is proficient in supporting the development, optimisation, and scale-up of a wide variety of conjugation processes.
Leveraging both mammalian and microbial manufacturing for the production of biologic intermediates, we can design and execute processes for a diverse range of bioconjugates, including but not limited to:
Antibody-Drug Conjugates (ADC)
Antibody Fragment-Drug Conjugates (ScFvDC)
Antibody-Oligonucleotide Conjugates (AOC)
Protein- and Peptide-Drug Conjugates
This combination of scientific breadth and technical expertise enables us to adapt development strategies to each modality — from conventional ADCs to next-generation conjugates — while ensuring scalability, safety, and regulatory compliance.
Technical Expertise in Conjugation
Our teams combine single-use conjugation systems with robust analytical and purification tools to deliver processes that are efficient, reproducible, and safe.
Site-specific conjugations to ensure batch-to-batch consistency, high payload homogeneity, and thermal stability
Expertise in diverse linker chemistries (maleimide, engineered cysteine, enzymatic, cleavable/non-cleavable)
Experience with multiple payload classes: small molecules, toxins, oligonucleotides, radionuclides
Scalable conjugation: from microgram development through to gram and clinical-scale GMP batches
Downstream recovery: high-containment chromatography, TFF for payload clearance
Analytical package including DAR determination, intact mass, peptide mapping, glycan analysis, stability testing

Streamline your ADC production with our comprehensive development & manufacturing services
With our unmatched expertise and capabilities, we seamlessly cover every stage of your antibody drug conjugate project, from antibody intermediate production to bioconjugation and GMP manufacturing of drug substance and drug product.
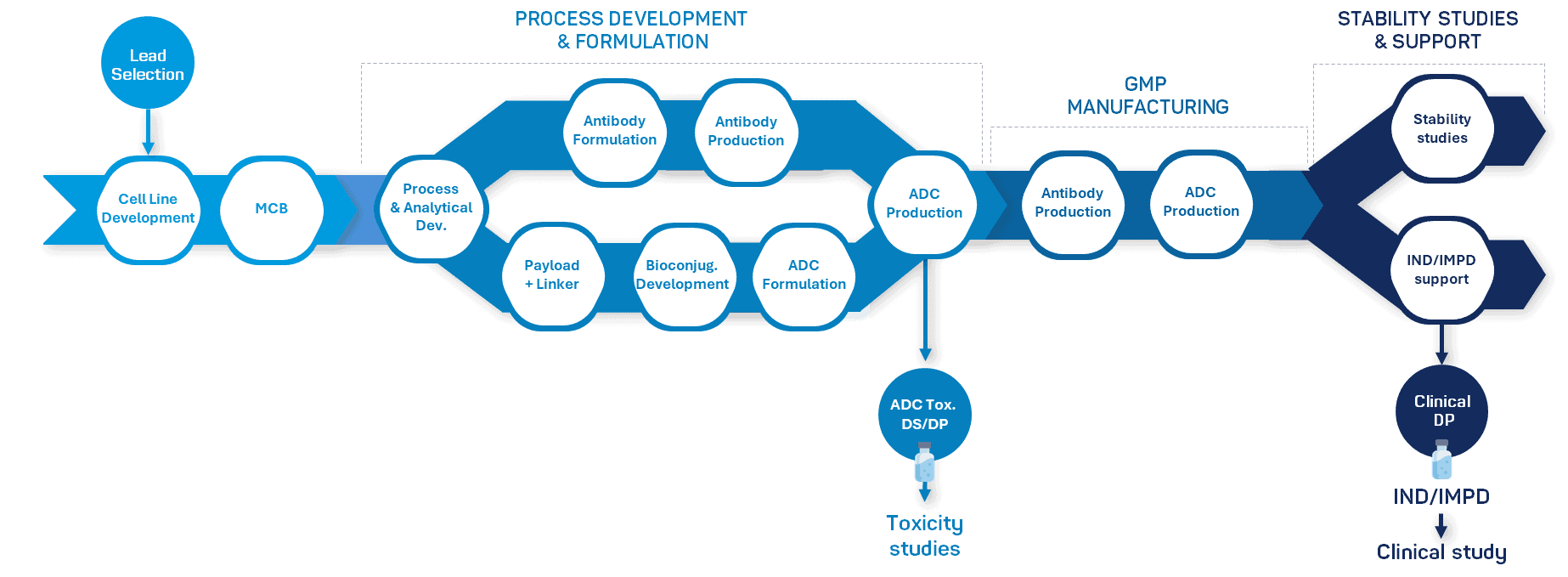
Integrated Development and GMP Transition

Bioconjugate development at Olon is engineered for regulatory alignment and smooth GMP transfer:
Development using scale-down GMP models to anticipate production behavior
Conjugation suites designed for high containment and operator safety
Integration with Olon Italy’s OEB6-certified Rodano facility for payload and linker manufacturing (OEL down to 10 ng/m³)
Dedicated QA/QC oversight ensuring compliance with EMA, FDA, and ICH requirements
This combination ensures your program can move confidently from R&D to clinical production.



