
Aseptic Fill & Finish
GTP Bioways provides Fill and Finish services for a wide range of active pharmaceutical ingredients including biologics, cytotoxic molecules and small molecules including nanomedicines, in a GMP-compliant environment.
About Us
We deliver drug product with full GMP-compliance
Our sterile fill finish facility was purpose-built to meet demand for small-scale, shorter sterile drug product development timelines, while consistently maintaining regulatory compliance for supply into Europe and the US.
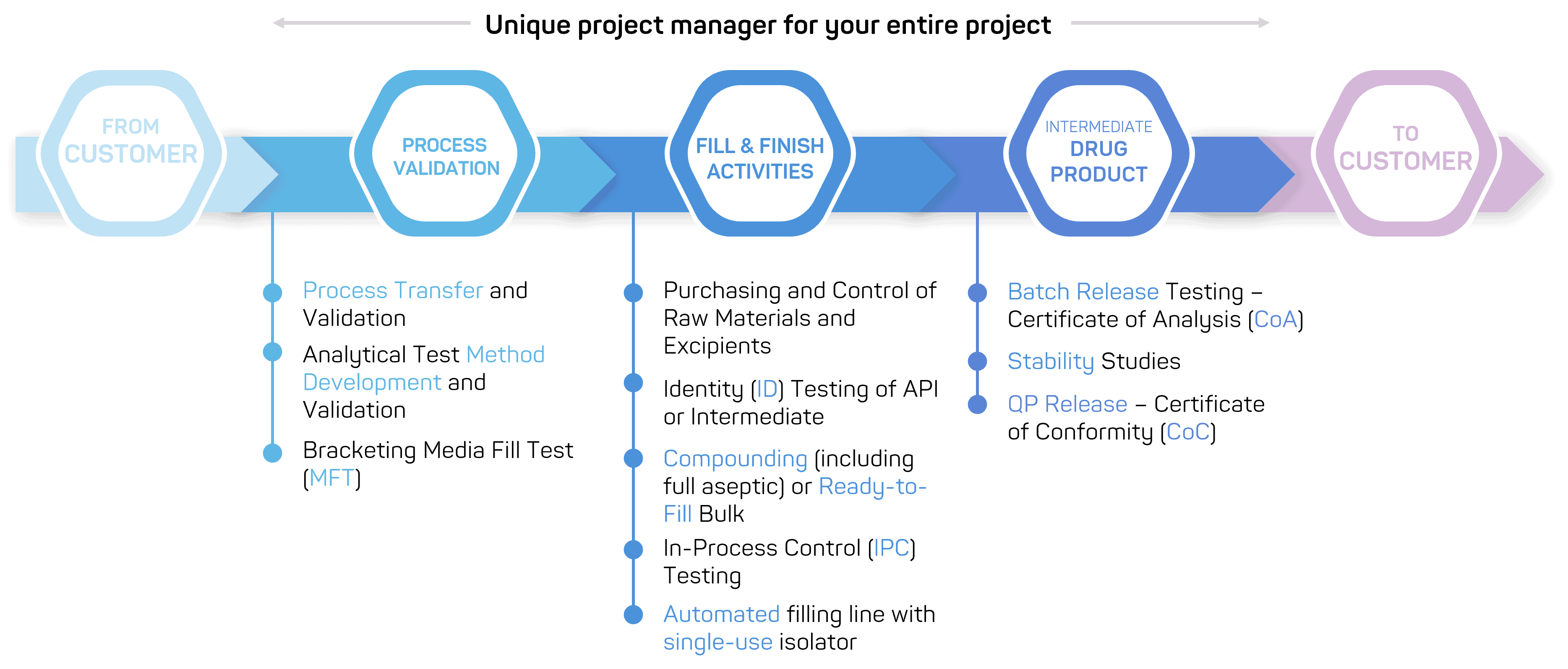
We perform sterile fill & finish activities for large and small molecule products under full global, GMP-compliance with finished dosage forms according to customer requirements and drug product specifications. We have authorizations for nearly all drug substances and are able to handle biotech products, nanocarriers, highly potent products and controlled substances.
Why you should work with GTP Bioways?
Choose our CDMO services to help you:
✔ Develop customized fill & finish approaches for specialized needs.
✔ Offer a unique aseptic filling line combining single-use isolator with automation (6-axis robot), which avoid cross contamination, limit human intervention and reduce downtime.
✔ Optimize the manufacturing process to your primary packaging (vials, syringes).
✔ Achieve easy scale up and optimal process transfer.
✔ Execute drug product manufacturing both through sterile filtration or full aseptic manufacturing for API which cannot be filtered.
✔ Perform testing services before and after fill & finish, including release testing & stability studies, and batch release by a Qualified Person.
10,000
Batches up to 10,000 units
20
100%
We offer a continuum of services dedicated to drug product manufacturing
Resources
Resources for Biopharmaceutical Companies
Aseptic fill & finish
GTP Bioways provides fill and finish services for a wide range of active pharmaceutical ingredients in a GMP-compliant environment.
Our manufacturing capacities
With production capacities dedicated to nanodrugs up to 60 kg, GTP Bioways is the ideal partner to cover your manufacturing needs during all development phases.
Knowledge center
We are happy to share with you our experience on innovative therapies development and manufacturing.