
Aseptic Fill & Finish Services for Biologics
Clinical to small commercial scale | Vials & Pre-filled syringes | EU GMP certified
About Us
Your CDMO Partner for Complex Biologics
At GTP Bioways, we provide end-to-end aseptic fill & finish services for your biologics — from formulation to EU GMP-certified filling in vials and pre-filled syringes.
Our agile facility is designed for clinical batches and small commercial production, with a strong focus on biologics requiring careful handling: proteins, ADCs, live microbial products, enzymes and more.
Why choose GTP Bioways for your fill & finish project?
EU GMP certified (France, ANSM)
Aseptic filling of vials (2–50 mL) & pre-filled syringes
Isolator-based technology
Dedicated formulation and compounding suites
Stability studies & QC services in-house
Integrated with upstream/downstream bioproduction
📍 Located in France – easy access from all major European hubs.
10,000
Batches up to 10,000 units
20
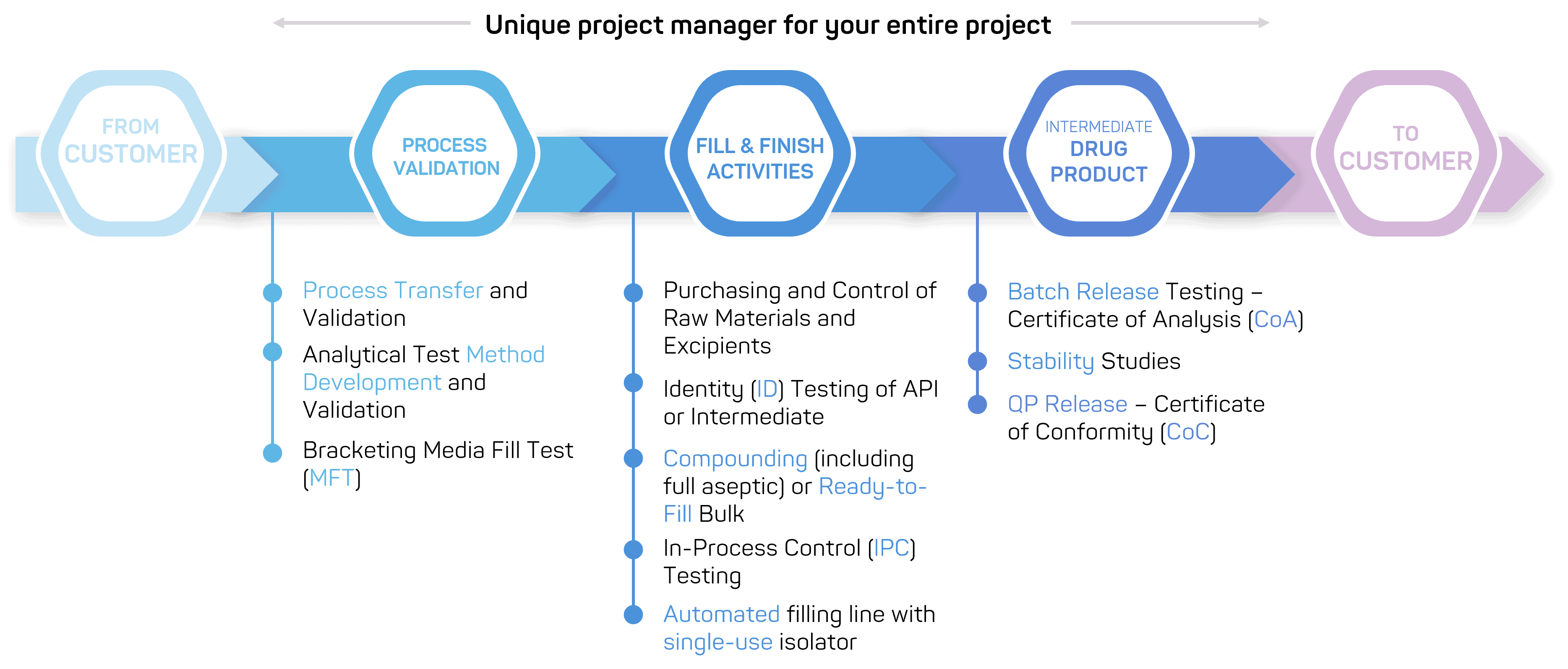
We offer a continuum of services dedicated to drug product manufacturing
Resources
Resources for Biopharmaceutical Companies
Aseptic fill & finish
GTP Bioways provides fill and finish services for a wide range of active pharmaceutical ingredients in a GMP-compliant environment.
Our manufacturing capacities
With production capacities dedicated to nanodrugs up to 60 kg, GTP Bioways is the ideal partner to cover your manufacturing needs during all development phases.
Knowledge center
We are happy to share with you our experience on innovative therapies development and manufacturing.